OCS Lung
The only FDA approved device for both standard and expanded* criteria donor lungs for transplantation
Often referred to as a “Lung-in-a-Box”, the OCS Lung is a revolutionary system that preserves donor organs.
The OCS acts as a miniature intensive care unit that keeps organs alive and healthy by maintaining them in a natural state that mimics the human body
so that organs can remain viable for transplant along the way to recipients.
The Benefits of OCS Lung
INSPIRE TRIAL RESULTS
The OCS Lung is FDA approved for use with standard criteria donor lungs

Improved post-transplant outcomes compared to cold storage

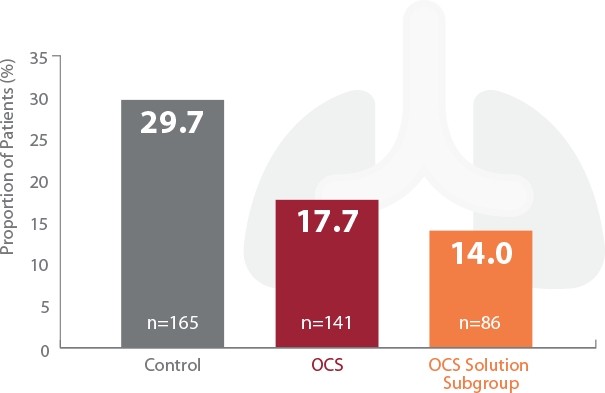
~50% reduction of primary graft dysfunction (PGD) grade 3†

Significantly expanded organ retrieval range while limiting ischemic time
Reduction of PGD3 with OCS
†PGD3 in the first 72 hours is a severe form of acute lung injury that is a major cause of early morbidity and mortality encountered after lung transplantation. TransMedics OCS Lung significantly reduced PGD3 vs control group in the INSPIRE Trial.
Significant Reduction of PGD3 within 72 Hours
EXPAND TRIAL RESULTS
The OCS Lung is FDA approved for use with expanded* criteria donor lungs

87% successful usage of donor lungs on OCS that had been rejected for transplant by centers using cold storage

Over 91% patient survival 1 year post transplant

Declined Lungs
UNOS data showed donor lungs used for the OCS Lung EXPAND Trial had been declined for transplantation on average 35 times by other transplant centers before reaching an OCS transplant center.
LONG-DISTANCE RETRIEVAL

20+ hours of cross-clamp time in a successful retrieval and transplant from Hawaii to North Carolina
*Expanded criteria is defined as donor lung pairs initially deemed unacceptable for procurement and transplantation based on limitations of cold static preservation.
Locate an OCS Lung Center Near you
The OCS Lung is FDA approved for use with standard and expanded* criteria donor lungs. The OCS Lung is CE marked and commercially available in Europe and Australia.
Massachusetts General Hospital
Main Campus
55 Fruit Street
Boston, MA 02114
(617) 726-2000
Houston Methodist Hospital
6565 Fannin St
Houston, TX 77030
Johns Hopkins Hospital
1800 Orleans St
Baltimore, MD 21287
(410) 955-5000
University of Minnesota Medical Center
909 Fulton St. SE
Minneapolis, MN 55455
(612) 273-8383
University of CA San Francisco Med Center
505 Parnassus Ave
San Francisco, CA 94143
(415) 353-1664
Duke University Medical Center
10 Duke Medicine Circle
Durham, NC 27710
(919) 372-3345
Temple University Hospital
3401 N Broad St
Philadelphia, PA 19140
(215) 707-2000
St. Joseph's (Dignity Health)
350 W Thomas Rd
Phoenix, AZ 85013
(602) 406-3000
University of Virginia (UVA)
1215 Lee St
Charlottesville, VA 22903
(434) 924-0000
UCLA Medical Center
757 Westwood Plaza
Los Angeles, CA 90095
(310) 825-9111
MCW/Froedtert
9200 W Wisconsin Ave
Milwaukee, WI 53226
(414) 805-3000
Stanford Medical Center
300 Pasteur Drive
Stanford, CA 94305
(650) 498-7878
Tampa General Hospital
1 Tampa General Circle
Tampa, FL 33606
(800) 505-7769
Henry Ford Hospital
2799 West Grand Blvd.
Detroit, MI 48202
(855) 858-7267
University of Chicago Medicine
5841 S. Maryland Avenue
Chicago, IL 60637
(800) 824-2282
University of Nebraska Medical Center
983285 Nebraska Medical Center
Omaha, NE 68198
(800) 401-4444
Baylor St. Luke’s Medical Center
6770 Bertner Ave.
Houston, Texas 77030
(832) 355-3000
Montefiore Medical Center
111 E 210th St 2nd Floor
The Bronx, NY 10467
(718) 920-2800
Houston Methodist Hospital
6565 Fannin Street
Houston, TX 77030
(713) 790-3311
Hannover Medical School (Medizinische Hochschule Hannover)
Carl-Neuberg-Straße 1, 30625 Hannover, Germany
Royal Papworth Hospital, Cambridge, UK
Papworth Rd, Cambridge CB2 0AY, United Kingdom
Padua University Hospital, Padua, Italy
Via Nicolò Giustiniani, 2, 35128 Padova PD, Italy
Policlinico Le Scotte Siena, Siena, Italy
Viale Mario Bracci, 16, 53100 Siena SI, Italy
Ospedale Niguarda, Milan, Italy
Piazza dell'Ospedale Maggiore, 3, 20161 Milano MI, Italy
Hôpital Nord, Marseille, France
278 Rue Saint-Pierre, 13005 Marseille, France
National Research Cardiac Surgery Center, Astana, Kazakhstan
Turan Ave 38, Nur-Sultan 020000, Kazakhstan
Hopitaux Universitaires de Strasbourg, France
1 Place de L Hôpital, 67000 Strasbourg, France
University Hospital Gasthuisberg, Leuven, Belgium
3001 Leuven, Belgium
The OCS Lung, OCS Heart and OCS Liver are all CE marked devices. The OCS Lung is an FDA approved device for standard and expanded1 criteria donor lungs. The OCS Heart is an FDA approved device for expanded2 criteria donor hearts. The OCS Liver is an FDA approved device for DCD3 and DBD donor Livers.


